Data support preserving access to accelerated approval drugs for the seriously ill
Commentary in the American Journal of Managed Care warns that restricting access to accelerated approval therapies has minimal budget implications, but poses significant harm to patients
March 30, 2021 (WASHINGTON, D.C.) The Partnership to Fight Chronic Disease (PFCD) today released, “Quantifying Impact of Accelerated Approval Drugs on Medicaid Spending: De Minimus Impact, Maximum Attention," an economic analysis examining the impact of the Food and Drug Administration’s accelerated approval pathway on Medicaid spending. A commentary based on the analysis was simultaneously published in the American Journal of Managed Care, "Limiting Access to Accelerated Approval Drugs: Costs and Consequences." The analysis, authored by PFCD Chairman and Chair of the Department of Health Policy & Management for the Rollins School of Public Health at Emory University Kenneth Thorpe and American Action Forum President Douglas Holtz-Eakin, found that drugs approved through the FDA’s accelerated approval pathway accounted for less than one percent of annual Medicaid spending between 2007 and 2018.
“Understandably, states are looking for effective means to manage their health care budgets. This was true before the COVID-19 pandemic and it is even more true today,” said Holtz-Eakin. “Through this analysis of Medicaid spending, we found that policies aimed at restricting access to therapies approved under the FDA’s accelerated approval pathway are not effective levers to address budget shortfalls. Given the unequivocally positive impact of these treatments on people living with diseases where there are no other treatment options, it is unacceptable for state and federal official to continue to pursue these ineffective policies.”
The accelerated approval pathway was initiated in 1992 to address unmet medical needs of oncology and HIV/AIDS patients. The pathway allows drugs for serious conditions to be approved by the FDA based on a surrogate endpoint — a measure that is reasonably likely to predict clinical benefit– with a post-marketing confirmatory study requirement to verify the predicted clinical benefit. In 2012, Congress modernized and enhanced the pathway to expand its use for rare diseases. The pathway has been credited with significant advances in the treatment of life-threatening diseases where patients have limited or no treatment options.
“The FDA accelerated approval pathway has given hope to countless patients struggling with life-threatening diseases, including cancer, HIV/AIDS and thousands of rare conditions,” said Annie Kennedy, chief of policy and advocacy at the EveryLife Foundation. “The burden of these diseases is so massive – from the individual patient and family burden to the broader societal and economic impact. It’s unthinkable to deprive patient communities the life-saving and life-altering benefits of these therapies that have potential to significantly alleviate those burdens. The findings published today demonstrate that therapies that meet the rigor of FDA approval through the accelerated approval pathway should not be candidates for Medicaid budget cuts.”
Medicines approved via accelerated approval meet the same standards for safety and efficacy as all FDA-approved medicines. Despite this, the accelerated approval pathway has come under scrutiny by public and private payers for its use of surrogate endpoints as the means to determine whether a drug works and because accelerated approval drugs are perceived as drivers of health care costs. Over the years, state Medicaid programs have inappropriately limited access to accelerated approval drugs and proposed excluding them from formularies, despite federal law requiring Medicaid to cover any FDA-approved treatment that meets the definition of a covered outpatient drug as defined in federal statute. For example, Massachusetts and Tennessee have formally requested waivers from the Centers for Medicare & Medicaid Services that would exempt them from this longstanding federal requirement.
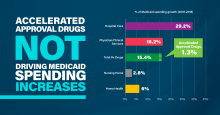
Thorpe and Holtz-Eakin’s analysis yielded the following key findings:
- Spending on drugs approved through the accelerated approval pathway accounted for less than one percent of annual Medicaid spending between 2007 and 2018.
- Spending on accelerated approval drugs remained steady at 0.6% to 0.8% beginning one year after the 2012 passage of the Food and Drug Safety and Innovation Act, which expanded use of the pathway to rare diseases.
- Hospital spending consumed the largest share of Medicaid spending year-over-year (34.5%), followed by physician and clinical services (11.7%) and all prescription drugs (8.9%).
- Contributors to the growth in overall Medicare spending from 2007 to 2018 included hospital spending (29.2%), all prescription drugs (16.7%), and physician and clinical services (16.2%).
- Accelerated approval drugs accounted for 1.3% of Medicaid spending growth from 2007 to 2018.
“These data clearly support preserving access to accelerated approval drugs for seriously ill patients within Medicaid programs,” stated Thorpe. “State schemes to avoid coverage requirements directly undermine Congress’s intent in establishing, and later enhancing, the accelerated approval pathway and raise serious concerns that certain Medicaid patients are not being offered equal access to life-saving medicines.”
For the full analysis of Medicaid spending on accelerated approval treatments and more information and resources on the issue, please visit: www.fightchronicdisease.org/resources/acceleratedapproval.
###
The Partnership to Fight Chronic Disease (PFCD) is an international coalition of patient, provider, community, business and labor groups, and health policy experts, committed to raising awareness of the number one cause of death, disability, and rising health care costs: chronic disease.
Media Contact:
Jennifer Burke
Jennifer.Burke@fightchronicdisease.org